Atomic Structure
Chlorine atom'>Atoms are the basic building blocks of everything around us. They come in different kinds, called elements, but each atom shares certain characteristics in common. All atoms have a dense central core called the atomic nucleus. Forming the nucleus are two kinds of particles: protons, which have a positive electrical charge, and neutrons, which have no charge. All atoms have at least one proton in their core, and the number of protons determines which kind of element an atom is. For example, an oxygen atom has 8 protons. If you were somehow able to change the proton number of this atom to 7, even if everything else remained the same, it would no longer be an oxygen atom, it would be nitrogen. For this reason, we list the different elements by their proton, or atomic, number. The periodic table of elements is a chart of all of the elements that have been discovered so far, in order by their atomic number.
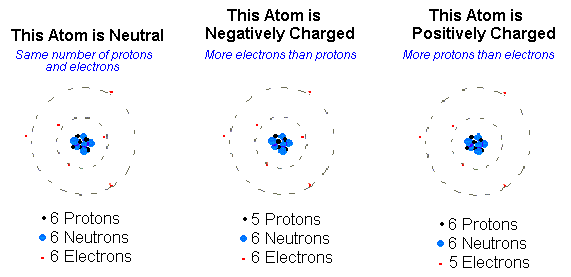
In addition to protons and neutrons, all atoms have electrons, negatively charged particles that move around in the space surrounding the positively-charged nuclear core. Electrons are usually depicted in drawings as much smaller than protons or neutrons because their mass is so much smaller. In fact, electron mass is so small that it is not counted in an atom’s mass. However, the charge strength of a single electron is equal to that of a single proton, and despite their small mass, electrons are important for balancing the charge of an atom. Unless specifically stated otherwise, atoms always have the same number of electrons as protons; therefore, you can find the electron number by looking at the atomic number. But unlike protons, the number of electrons can and does change without affecting the kind of element an atom is!
The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915. Answers for electrically charged atom crossword clue. Search for crossword clues found in the Daily Celebrity, NY Times, Daily Mirror, Telegraph and major publications. Find clues for electrically charged atom or most any crossword answer or clues for crossword answers.
We now know how to find the number of protons and the number of electrons for a given atom, but what about neutrons? How many neutrons do atoms of a given element have? It is NOT always the same as the number of protons and electrons. For example, hydrogen has one proton and one electron, but it doesn’t have any neutrons at all! We determine this by looking at the atomic mass. Even though an atom is so small it would take almost a million for you to see even a tiny dot on your computer screen, each tiny atom definitely has mass and occupies space. This mass comes from the nucleus. Each proton and neutron has about the same amount of mass, measured in daltons, or atomic mass units (amus). Because the unit of measure is defined by one proton, 1 proton = 1 neutron = 1 dalton = 1 amu. Electrons do have some mass, but it is almost 2000 times less than the mass of a proton. There aren’t enough electrons in any of the atoms we know about to affect the total mass; therefore, the total mass is equal to the sum of the protons and the neutrons in an atom.
Because we can find the number of protons and the atomic mass of an atom by looking at its element information in the periodic table, we can calculate the number of neutrons in that atom by subtracting the number of protons from the atomic mass.
When the number of neutrons is different for individual atoms of the same element, each atom is called an isotope. When you read a periodic table, the atomic mass listed is the average atomic mass for all of the isotopes of that element found in nature. For example, carbon has an atomic mass of 12.01 in the periodic table. Carbon can’t have 6.01 neutrons because you can’t have part of a neutron. The value exceeds 6 because, while most carbon atoms have 6 neutrons, some carbon atoms are found with 7 neutrons and others with 8 neutrons. For our purposes, we round the atomic mass to the nearest whole number to calculate the number of neutrons.
Atomic Structure
This video illustrates how atoms and their components work together.
Valence Electrons
Now that you’ve had a chance to work with atoms in general, let’s dig a little deeper. Electrons remain in an atom because of their attraction to the protons’ positive charge, but they are not as tightly associated with the atom as either protons or neutrons. Electrons are complicated particles because they have a lot of space to occupy in an atom, and yet they are also tied to a specific area within that atom. Although the drawings we have been working with show the nucleus as a medium-sized, visible object in the center of an atom, it is actually very tiny, and most of an atom is the space around the nucleus in which the electrons move.
Because of their shared negative charge, electrons repel one another if they get too close. At the same time, electrons are attracted to the positive charge of the nucleus. The details of the energy and position of electrons can get really complicated, but we will focus only on what we need to understand to study the molecules of life.
Sulfur atom'>Electrons are arranged in energy shells (also known as electron shells) around the atomic nucleus. Although electrons have plenty of space, they all want to be closest to the positive nuclear charge that is attracting them. At the same time, the electrons repel one another because of their negative charge, and only a few can get close to the nucleus at any given time. Practically speaking, only two electrons can fit in the three-dimensional space closest to the nucleus. This space is called the first energy shell. If there are three electrons in an atom, the first two will be found in the first energy shell. The third electron will have to settle for the second energy shell, a three-dimensional space a little farther from the nucleus, where it will be alone. In this example, the lone electron is called a valence electron, and the outermost energy shell that contains any electrons is called the valence shell.
Aluminum atom'>The second energy shell is big enough to hold as many as eight electrons, grouped in pairs inside four electron orbitals, or spaces where electrons spend most of their time. This means if there is only one electron in the second energy shell, there is a lot of extra space left.
When an energy shell is incompletely filled, the electron(s) in that shell are not as stable and are more likely to react. For this reason, atoms tend to react with other atoms in ways that will fill or empty their valence shell to gain the stability of a full outermost energy shell. Atoms can do this by gaining or losing electrons to become ions or by sharing electrons with other atoms to form stable associations.
Using electron number and energy shells, we can determine the number of valence electrons for any given atom and its expected level of reactivity. As you work with the example below, you should remember that although we draw energy shells as circles around an atomic nucleus, this is not meant to represent an actual electron path. The concentric circle style of drawing energy shells is meant to represent the average distance electrons in that energy shell are orbiting the nucleus. In reality, electrons do not move in a circular orbit as depicted in the drawing, but travel much more complicated pathways around an atomic nucleus.
Build an Atom
Use this activity to practice reading the periodic table to create several atoms.
What is the Valence?
In this activity, you will calculate the number of valence electrons in atoms using the periodic table of elements.
Comparing structural effects on acidity
Generally, negative charges are stabilized by allowing the charge to delocalize over a bigger space or over more atoms. Typically, a molecule that holds the negative charge over several atoms will be more stable than a molecule that localizes the full charge on a single atom. The main features that determine the stability of a negative charge are the following:
- The electronegativity of the atom on which the negative charge is located
- The size of the atom on which the negative charge is located
- Stabilization of the negative charge through resonance
- Stabilization of the negative charge by adjacent electronegative atoms
I'll look at each of these factors individually.
Electronegativity of negatively charged atom
Simply put, negative charges prefer to rest on more electronegative elements (see here for more on electronegativity) than on more electropositive elements (like carbon). That's why water is more acidic than ammonia (NH3), because oxygen is more electronegative than nitrogen. Similarly, both water and ammonia are more acidic than methane (CH4) since carbon is more electropositive than nitrogen or oxygen.
Size of negatively-charged atom
Atoms Crossword
The size of the atom on which the negative charge rests also effects the stability. As a general rule, negative charges prefer to rest on larger atoms, as the charge can spread over a much larger region of space (making it more stable) than when the charge is localized in a much smaller space on a smaller atom. Generally, this preference for placing the charge on a larger atom trumps electronegativity considerations. For example, HI is more acidic than HF, even though based on the last argument about electronegativity you might suspect that F- would be more stable than I- because fluorine is more electronegative than iodine (and thus HF would be a stronger acid than HI). This turns out not to be the case. Iodine is so much bigger than fluorine that the charge is more stable on this larger atom. Thus, the trends for acidity of the hydrohalic acids are as follows: HI is strongest, followed by HBr, HCl, and finally HF.
Resonance stabilization
One of the most important effects on the acidity of a molecule is whether the conjugate base anion can be stabilized by resonance. Phenol, for example (shown below), is about 1,000,000 times stronger an acid than cyclohexanol because the conjugate base of phenol is much more stable than the conjugate base of cyclohexanol. This added stability of the phenol conjugate base arises because this anion can delocalize the negative charge throughout the ring through resonance, effectively stabilizing it. The conjugate base of cyclohexanol has no resonance structures to stabilize the charge and so is less stable.
Charged Atom Crossword
| Electons on phenol are stabilized by their delocalization throughout the ring (shown below); cyclohexane has no electron delocalization, and thus is a much weaker acid. |
Charged Atom Or Group Of Atoms
Neighboring electronegative groups
Charged Atom Examples
The presence of electronegative groups near a hydrogen also makes it more acidic. You can see why this is so by using the same analysis as before. That is, somehow the presence of these electronegative atoms must stabilize the conjugate base anion. For example, substituting the electronegative atom chlorine for hydrogen in acetic acid makes the molecule about 100 times more acidic (recall that the pKa scale is defined logarithmically). This increase in acidity is due to the electronegative chlorine atom pulling some of the electron density away from the oxygens, making the oxygens not having to bear all of the negative charge by themselves.
